Löytyi myös suomenkielisenä:On. Tässä valmistaja https://www.efoy.com/
https://hydrocell.fi/polttokennot/efoy/
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
Löytyi myös suomenkielisenä:On. Tässä valmistaja https://www.efoy.com/


Ja tuo kuvan vehje tuottaa jotain 100-50 kW.Koska omavaraistelu kiinnostaa, ja varsinkin metanolilla sähkön tuottaminen, niin pakkohan se oli työntää päänsä syvemmälle kaninkoloon tässä polttokenno asiassa. Tälläinen tuli vastaan:

Kukaan ei ihmetellyt, kun tietoliikenne katkesi Aapeli-myrskyn kourissa ympäri Pirkanmaata, mutta Parkanossa puhelimet toimivat – salaisuus löytyy kennosta, jota on käytetty Nasan raketeissa
Parkanon taajamassa ja maaseutukaupungin länsiosissa kukaan ei osannut ihmetellä, miten matkapuhelimet pelasivat moitteetta Aapeli-myrskyn pitkän sähkökatkon aikana.www.aamulehti.fi
Ilmeisesti tekniikka on ottanut roimia harppauksia, koska lähemmäs kymmen vuotta sitten nuo linkkimastot sähköistettiin (ainakin virka-apu harjoituksissa) verkkosähkön lamaannuttua tämänkaltaisilla laitteilla:
Katso liite: 50937
muoks: löysin etsimäni pienellä vaivannäöllä
Since World War II, the U.S. Army has used approximately 20 times more energy per soldier while reducing the number of soldiers deployed. This undoubtedly will continue in the future as new capabilities with higher-power weapons, novel sensors and robots, and advanced computing are envisioned. This highlights the importance of energy supply and management for the future battlefield.
When considering Army maneuver, one key objective is minimizing the number of trucks carrying materials to the battlefield. As Army Futures Command explores future autonomous vehicles and active protective systems, the concern becomes less about lost lives (which was a major factor in Iraq and Afghanistan) and more about lost transport vehicles, fuel, water, munitions and other supplies, as autonomous systems would not require the physical presence of a human.
Looking at the transportation fuels used today, diesel, JP8 and biodiesel clearly have the highest volumetric energy density. And this is the most important measurement, as supply trucks generally “cube out” before they “weigh out.

Some sodium battery developers are using activated carbon for the anode, which holds sodium ions in its pores. "But you need to use high-grade activated carbon, which is very expensive and not easy to produce," Sun says.
Graphite, which is the anode material in lithium-ion batteries, is a lower cost option. However, sodium ions do not move efficiently between the stack of graphene sheets that make up graphite. Researchers used to think this was because sodium ions are bigger than lithium ions, but turns out even-bigger potassium ions can move in and out easily in graphite, Sun says. "Now we think it's the surface chemistry of graphene layers and the electronic structure that cannot accommodate sodium ions."
He and his colleagues have come up with a new graphite-like material that overcomes these issues. To make it, they grow a single sheet of graphene on copper foil and attach a single layer of benzene molecules to its top surface. They grow many such graphene sheets and stack them to make a layer cake of graphene held apart by benzene molecules.
The benzene layer increases the spacing between the layers to allow sodium ions to enter and exit easily. They also create defects on the graphene surface that as as active reaction sites to adsorb the ions. Plus, benzene has chemical groups that bind strongly with sodium ions.
This seemingly simple strategy boosts the material's sodium ion-storing capacity drastically. The researchers' calculations show that the capacity matches that of graphite's capacity for lithium. Graphite's capacity for sodium ions is typically about 35 milliAmpere-hours per gram, but the new material can hold over 330 mAh/g, about the same as graphite's lithium-storing capacity.

 spectrum.ieee.org
spectrum.ieee.org
British engineering and aerospace giant Rolls-Royce has secured funding to build nuclear power stations based on small modular reactor (SMR) technology.
A consortium of BNF Resources UK LTD, Exelon Generatuion Lt and Roll-Royce Group will invest £195m roughly over a three-year period. This cash injection will allow the companies to qualify for a £210m grant from the British government, specifically the UK Research and Innovation Funding.
The path forward includes Rolls Royce entering the UK Generic Design Assessment process and closing in on sites for the factories to build the modules that will allow for on-site assemply of the power plants.
The funding could see four SMRs built based on nuclear submarine technology. Rolls Royce said a SMR power station will be the size of two football pitches and power circa one million homes.

Supercapacitors are definitely not the same as batteries, we all know that. They tend to have a very low operating voltage, and due to their operating principle of storing charge on parallel plates, their discharge curve is quite unfriendly for modern microcontroller devices. Energy storage efficiency per unit volume is also low compared with modern lithium polymer (LiPo) batteries so all in all they don’t look all that useful for many of our projects. However, as [Andreas Spiess’] latest video demonstrates, they do have some redeeming features that might make them useful for certain embedded applications.
The low operating voltage initially looks like an issue for devices operating at a typical 3.3V, and it’s tempting to simply wire a few in series and roll with it. But as [Andreas] explains in his typically clear manner, it would be necessary to have a complex power stage, operating in buck mode with capacitor voltage above the required level, and in boost mode when it heads below. Too complex – it’s much easier to simply stick with a low voltage bank of paralleled supercaps, and just operate always in boost mode. Even doing this, you’re not realistically going to get more than a handful of hours operating voltage with an always active device.
So why bother at all with supercaps, surely using a LiPo is so much easier and better? In many cases the answer is definitely a yes. But LiPo cells must not be charged in freezing temperatures (apart from certain special low temp products), else the cell can rapidly be destroyed due to lithium metal deposition at the anode. Also you need to be careful charging them, especially when they’re heavily discharged, as they are easily damaged without the proper treatment. LiPo cells operate based on chemical principles – lithium ions literally have to move around inside the structure, and eventually the battery will wear out.
Supercapacitors have the advantage of very long life (but sometimes, they do leak) much more aggressive charging and discharging behaviours and will operate down to very low temperatures. This makes them very useful when a large amount of power is available sporadically (for super fast charge cycles) or in places where temperatures stay persistently very low, such as up a mountain were solar will work, albeit slowly, but LiPo batteries will definitely not be suitable.
Other battery chemistries are available, such as Lithium Iron Phosphate which can tolerate the cold. Also you can always just insulate the battery with an integrated heater and preheat the battery to a safe charging temperature as well. So, just like everything with electronics, it’s important to choose the correct parts for your application, and it all starts with the power source. Supercapacitors might just hit an appropriate price/performance point for that special application you had in mind.
Supercapacitors aren’t really suitable for many applications, like powering an eBike or running your laptop, but hey, they did it anyway.
Pumped hydro storage is one of the oldest grid storage technologies, and one of the most widely deployed, too. The concept is simple – use excess energy to pump a lot of water up high, then run it back through a turbine when you want to get the energy back later.
With the rise in renewable energy deployments around the world, there is much interest in finding ways to store energy from these often-intermittent sources. Traditional pumped hydro can help, but there is only so much suitable land to work with.
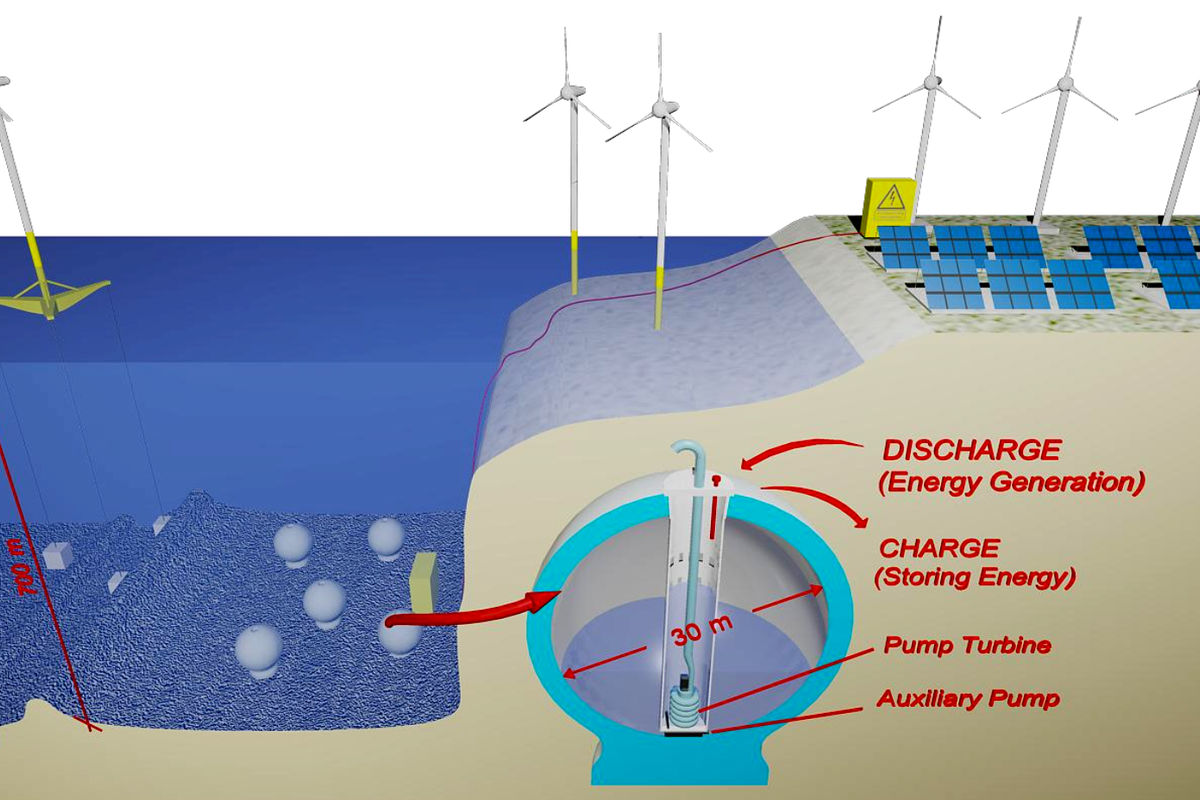
The general idea is to have a closed vessel sitting on the seafloor. Surplus energy is then used to pump water out of this vessel, leaving the inside at a near-vacuum. When it’s desired to recover energy from the system, water can be allowed to flow back into the vessel under the pressure generated by the seawater above. As the vessel is filled, the water flowing in turns a turbine, generating electricity in just the same way as a traditional pumped hydro system.
It’s a dedicated hacker who has the patience to build an engine from scratch. And it’s a borderline obsessed hacker who does it twice. [Meanwhile In the Garage] is of the second ilk, and in the video below the break, he takes a failed engine design and musters up the oomph to get it running.
The whole build began with an idea for a different kind of intake and exhaust valve. [Meanwhile In the Garage] dreamed up a design that does away with the traditional poppet valve. Instead of valves that open by being pushed away from their seat by a camshaft, this design uses a cylinder that is scooped so that as it rotates, its ports are exposed to either the intake or the exhaust.

Physicists first suspected more than a century ago that the fusing of hydrogen into helium powers the sun. It took researchers many years to unravel the secrets by which lighter elements are smashed together into heavier ones inside stars, releasing energy in the process. And scientists and engineers have continued to study the sun’s fusion process in hopes of one day using nuclear fusion to generate heat or electricity. But the prospect of meeting our energy needs this way remains elusive.
The extraction of energy from nuclear fission, by contrast, happened relatively quickly. Fission in uranium was discovered in 1938, in Germany, and it was only four years until the first nuclear “pile” was constructed in Chicago, in 1942.
There are currently about 440 fission reactors operating worldwide, which together can generate about 400 gigawatts of power with zero carbon emissions. Yet these fission plants, for all their value, have considerable downsides. The enriched uranium fuel they use must be kept secure. Devastating accidents, like the ones at Chernobyl in Ukraine and Fukushima in Japan, can leave areas uninhabitable. Fission waste by-products need to be disposed of safely, and they remain radioactive for thousands of years. Consequently, governments, universities, and companies have long looked to fusion to remedy these ills.
Among those interested parties is NASA.
 spectrum.ieee.org
spectrum.ieee.org
Quaise Energy plans to use millimeter-wave drilling systems that can reach depths where geothermal energy is virtually unlimited. The firm will drill with microwaves in order to go deeper than ever before.
On its website, Quaise Emergy describes the technology as follows: “Our gyrotron-powered drilling platform vaporizes boreholes through rock and provides access to deep geothermal heat without complex downhole equipment. Based on breakthrough fusion research and well-established drilling practices, we are developing a radical new approach to ultra-deep drilling.”
Better yet, the firm does not plan on producing new geothermal plants from scratch but is rather looking at repowering old oil and gas plants. This will be more cost-efficient and also allow the workforce from these plants to join the clean energy transition.
Finally, Quaise Energy claims that deep geothermal uses less than 1 percent of the land and materials of other renewables, making it the most convenient and profitable choice toward a clean energy future. The company has already raised $63 million indicating it is well on its way to making ubiquitous access to geothermal a viable option.


Verrokiksi; jos Kaipola toimisi yhä täydellä teholla eikä Jämsänkoski olisi lakossa, ne veisivät yhdessä noin 2/3 tuosta tämänhetkisestä tehosta.Nonni, lauantaina alkoi OL3 tuottamaan lamppuun valkeaa. On osa maamme kokonaisturvallisuutta
TVO - OL3-tuotanto
www.tvo.fi
Joo, tämänhetkinen tehohan on vain vähän isompi kuin se, mitä laitos itse näyttäis kuluttavan. Harjoittelevat vielä erilaisia pikasulkuja ym. Täydelle höyrylle on suunnitelma vasta kesästä alkaen. Saattaa parantaa tarkenemista noustalvena.Verrokiksi; jos Kaipola toimisi yhä täydellä teholla eikä Jämsänkoski olisi lakossa, ne veisivät yhdessä noin 2/3 tuosta tämänhetkisestä tehosta.
Yli puolet suvustani on ollut Kaipolassa, isäni jopa sitä rakentamassa.Joo, tämänhetkinen tehohan on vain vähän isompi kuin se, mitä laitos itse näyttäis kuluttavan. Harjoittelevat vielä erilaisia pikasulkuja ym. Täydelle höyrylle on suunnitelma vasta kesästä alkaen. Saattaa parantaa tarkenemista noustalvena.
Enpäs muuten tiedäkään Kaipolan muinaista sähkönkulutusta enkä tuottoa. Helppo kuitenkin uskoa, että yli 50 MW menee. Olin siellä konekorjaamolla harjoitteluhommissa -70 -luvulla ja mm. kokoamassa voimalaitoksen turbiini-generaattori -yhdistelmää Siemensin ukkojen kanssa.
Tesla teamed up with SpaceX to provide coverage expansion for its Starlink services to help provide an alternative internet infrastructure.
Volunteers across the Giga Berlin and Germany Service team responded quickly on Sunday to test, configure, pack and ship several hundred Starlink units which have already been gratefully received by Ukraine’s Digital Transformation Minister. In true Tesla fashion, the solution has been put together in less than 3 days.
On top of this the Energy team supplemented the Starlink roll out with a fleet Powerwalls. The system included PV inverters given by our Certified installer network, pre-made DC cables given by one of our Supercharger Installation Partners and AC cables made out of scrap from Giga Berlin. All of it assembled by a team of (40+) volunteers from across the EMEA organization, committed to doing what they can to support.
Very cool, but ... it's also a bit problematic, because of the lithium-ion batteries. It is a solution, but it is also a risky solution because of the fire risk. However, it can provide stored power into the energy infrastructure as that is it's main design function, to be the backup for the emergencies. To get it back up and running, you'll need the energy sources. Therefore, as a wartime solution it's a bit meh.
Just a few hours before massed Russian troops and missiles surged over borders with deadly force last month, Ukraine’s grid operator opened a series of high-voltage breakers, disconnecting the nation’s grid from those of Belarus, Russia, and the rest of the giant UPS/IPS synchronous AC power zone controlled from Moscow. It was supposed to be a 72-hour test, and going ahead under the tense circumstances was a bold and risky gambit, admits Ukrenergo CEO Volodymyr Kudrytskyi.
“We heard a lot of opinions in the expert community, as well as among politicians that it is very dangerous to disconnect from Russia and Belarus, that the Ukrainian energy system will not be able to function independently for a long time,” recalled Kudrytskyi in an interview published yesterday by nonprofit Kyiv-based news outlet Ukrayinska Pravda.
Ukraine’s grid held, even as Russia damaged substations, lines, and generators—a remarkable and heroic (though largely unheralded) achievement. As recently as November, generation shortfalls had prompted Kyiv’s mayor to voice fears of rolling blackouts. Tight coal and gas reserves continued to bite throughout the winter.

 spectrum.ieee.org
spectrum.ieee.org
keskimmäisestä artikkelistaHarjoittelua on ollut, mutta onkohan missään skenaariossa todella otettu huomioon Venäjän mahdollista aggressiota?
Olkiluoto 3 tuo omavaraisuuteen helpotusta. Turpeen käytön mahdollistaminen olisi hyväksi. Loviisa on ongelma polttoaineen osalta. Mitä puuttuu 5-10%?
Olisimme kaivanneet harjoitukseen vielä kovempaa prässiä ja painetilannetta, sillä näin valtavassa häiriötilanteessa paine viestinnän ja tiedotuksen osalta olisi jatkuvaa. Jouduimme pohtimaan myös resursointikysymyksiä sekä tietysti henkilöstön tiedottamista tilanteessa, jossa sähköiset tiedotusvälineet eivät toimi.
Algae are terrific at fixing carbon. Like plants, they absorb large quantities of carbon dioxide from the atmosphere and convert it into sugars via photosynthesis. Plus, the slimy green stuff grows faster than trees, and can grow on land and in water unsuitable for food crops. All these are reasons why algae are considered an important tool to address climate change.
That is, until you try to cultivate algae at large scales. Producing large enough amounts of algae to be cost-effective has proven immensely challenging and kept algae biofuels from reaching the market. But now researchers have tapped into artificial intelligence to grow algae at a record pace that could bring down algae fuel cost.
By using machine learning to precisely tune the growth of blue-green algae, also known as cyanobacteria, the Texas A&M University team is able to produce over 43 grams per square meter a day in an outdoor experimental setup. That’s about twice the 25 grams/m2/day target recently released by the U.S. Department of Energy. It’s “absolutely without any question the highest reported outdoor algae growth speed and productivity,” says Joshua Yuan, professor and chair of synthetic biology and renewable products at TAMU.

 spectrum.ieee.org
spectrum.ieee.org
The new high yield would bring the algae selling price to US $309 per tonne ($281 per tonne), less than a quarter of the selling price of algae cultivated in 2019 using state-of-the-art open-pond methods. Yuan cautiously estimates that his team's process has the potential to bring the algal biofuel price to below $5 per gallon ($1.32 per liter) from today’s exorbitant $33 per gallon ($8.71 per liter). That's slightly higher than corn ethanol, which is $2 to $4 per gallon (53 cents to $1.06 per liter) and is “already getting competitive with fossil fuels in California, as we can link to carbon capture to bring carbon credits,” he says. “By combining carbon credit and lowering fuel price, we can be competitive with fossil fuels. Furthermore, this fuel price is competitive in overseas markets, like China, where fuel prices are much higher.”
In early February, the DOE announced $19 million of funding for projects that can boost the capacity of algal systems to capture carbon dioxide, as a way to both reduce carbon emissions and produce algae for biofuels and other products. “Theoretical algae yield is huge, much better than land-based plants, but there are a few major limitations,” Yuan says—namely, growing and harvesting algae.
In both closed reactors and open-pond systems, as algae grows and its concentration increases on the surface, it blocks light from reaching the cells underneath, slowing down growth. Plus, harvesting it is inefficient and costly. Traditional ways to separate algae from water such as centrifugation, filtration, and chemical flocculation, can make up as much as half of the total energy use and third of the total costs of producing algae.
