Tässä ei mene faktantarkastajien perustaktiikka läpi. Artikkelin metodiikkaosiosta löytyy laboratoriotuloksen vaatimus sille, että tapaus on hyväksytty tarkasteluun:
https://www.medrxiv.org/content/10.1101/2021.08.30.21262866v1.full
SARS-CoV-2 mRNA Vaccination-Associated Myocarditis in Children Ages 12-17: A Stratified National Database Analysis
"METHODS
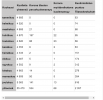
We searched the Vaccine Adverse Event Reporting System (VAERS) data for females and males ages 12-17 in reports processed from 1/1/2021 through 6/18/2021 with diagnoses of “myocarditis,” “pericarditis,” “myopericarditis” or “chest pain” in the symptom notes
and required the term “troponin” in the laboratory data. We defined a CAE using the CDC working case definition for a probable case.[
2] Specifically, the symptom of “chest pain” required at least one of the following: diagnosis of myocarditis, peri- or myopericarditis, acute myocardial infarction; elevated troponin; abnormal electrocardiogram (EKG), abnormal echocardiogram (ECHO), or cardiac MRI (cMRI) findings consistent with myocarditis (as defined in Supplement1). Cases and hospitalizations with an unknown dose number were assigned to dose 1 or dose 2 in the same proportion as the known doses: 15% occurred following dose 1 and 85% occurred following dose 2.
To compute crude rates per million for doses 1 and 2, our denominators included all children with at least 1 dose of any vaccination and all fully vaccinated children, respectively, as of 6/11/2021[
6] to accommodate both reporting lag and a pre-defined 7-day risk window, consistent with the CDC’s analysis. 95% Poisson confidence intervals were calculated for these rates."
-----
Tukeva lähde:
https://heart.bmj.com/content/106/15/1127
COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis?
"Cardiac troponin levels are significantly higher in patients with more severe infections, patients admitted to intensive care units or in those who have died. In the setting of COVID-19, myocardial injury, defined by an increased troponin level, occurs especially due to non-ischaemic myocardial processes, including severe respiratory infection with hypoxia, sepsis, systemic inflammation, pulmonary thrombosis and embolism, cardiac adrenergic hyperstimulation during cytokine storm syndrome, and myocarditis."
----
Voi Pfizer-yhtiö raukkaa, taas ilkeämielisen anti-rokoteaktivistien salaliittokonglomeraatin uhri. Harmi että nuo haittailmoitusrekisterit on tullut aikanaan rakennettua, muuten voitaisiin luottaa suoraan valmistajien ilmoituksiin ja valmistajien tekemään turvallisuusseurantaan omista tuotteistaan. Pitäisi lopettaa Suomestakin tuo FIMEA:n järjestelmä niin saataisiin tämä mRNA-rokotekampanja vietyä 2-vuotiaisiin asti läpi ilman hämminkiä.
Tämä meta-analyysi varmentaa laajemmalla tietopohjalla mRNA-rokotteiden aiheuttavan erityisesti nuorille aikuisille huomattavasti enemmän haittavaikutuksia kuin perinteisemmät rokotetekniikat. Ja sitten Pfizerin Comirnartyllä, joita meidän lapsille aggressiivisesti tarjotaan, lapsilla ja nuorilla on vielä enemmän haittavaikutuksia kuin niillä nuorilla aikuisilla.
Eri maiden haittailmoitusrekisterit olivat vertailun vuoksi meta-analyysissä mukana ja huomattiin, että koronarokotteisiin liittyviä haittailmoituksia on niissä suhteellisesti vähemmän kuin asiallisesti tehdyissä tutkimuksissa ilmaantuvuustasot antaisivat olettaa. Vaihdellen eri maissa voimakkaasti.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8315897/
Evaluation of the safety profile of COVID-19 vaccines: a rapid review
"
Results
A total of 87 publications with safety data from clinical trials and post-authorization studies of 19 COVID-19 vaccines on 6 different platforms were included. The pooled rates of local and systemic reactions were significantly lower among inactivated vaccines (23.7%, 21.0%), protein subunit vaccines (33.0%, 22.3%), and DNA vaccines (39.5%, 29.3%), compared to RNA vaccines (89.4%, 83.3%), non-replicating vector vaccines (55.9%, 66.3%), and virus-like particle vaccines (100.0%, 78.9%). Solicited injection-site pain was the most common local reactions, and fatigue and headache were the most common systemic reactions. The frequency of vaccine-related serious adverse events was low (< 0.1%) and balanced between treatment groups. Vaccine platforms and age groups of vaccine recipients accounted for much of the heterogeneity in safety profiles between COVID-19 vaccines. Reporting rates of adverse events from post-authorization observational studies were similar to results from clinical trials. Crude reporting rates of adverse events from post-authorization safety monitoring (passive surveillance) were lower than in clinical trials and varied between countries."
"
Age subgroup analysis based on data from clinical trials
The rate of the most common solicited symptoms was significantly higher among younger adults compared to the elderly (Additional file 1: Table S11). RNA vaccines had significantly higher rate of most common solicited reactions (e.g., injection-site pain, fatigue, headache) among younger adults compared to the other 5 platforms, regardless of the grades of adverse reactions (except overall injection-site pain which was also quite high for virus-like particle vaccines) (Additional file
1: Figures S4-S6). Meanwhile, the highest risk of these common systemic reactions (including fever) was observed in RNA vaccine recipients in this age group, compared to controls (Additional file
1: Table S12). While the highest rate of fever was shown in virus-like particle vaccines (Additional file
1: Figure S7). Differences between vaccine platforms and age groups of vaccine recipients accounted for much of the heterogeneity in safety profiles between COVID-19 vaccines (Additional file
1: Table S13).
In addition, the rate of AEFI after CoronaVac was less frequent in children and adolescents than in younger adults, whereas the reverse was found with BNT162b2 (Additional file
1: Table S7)."
---
Sitten tuo tohtori Lee Merritt U.S.Navy -taustallaan puhuu kyllä myös Yhdysvaltain asevoimien omista rekistereistä, jotka näyttää huolestuttavia asioita.
---
Laitetaan tähän vielä loppukevennys. Jos lapsesi sattuukin olemaan tyttö, niin sydäntulehdusriski ei ole suurempi kuin koronan luomuna sairastaessa. Sen sijaan ulkonäkö voi vähän kärsiä:
https://www.dailyveracity.com/2021/09/01/woman-has-strange-reaction-to-pfizer-vaccine/
Eikö ollutkin hauska




ja varsinkin se ukko siellä alakuvissa, hilarious!!!!

www.kymensanomat.fi